Is aluminum in vaccines safe, or toxic? Why is aluminum in vaccines?
——————————————————
Here is the Toxicological Profile on Aluminum, prepared by the Agency for Toxic Substances and Disease Registry, or ATSDR.
If you were to read through the 300+ pages of this report, you would find that it’s full of information on scientific research which aims to determine the effect of aluminum exposure on the body – whether ingested, inhaled, etc., at what exposure limit it has been found safe or harmful, and the scientific research which supports the proposed safe limits. You would also find that the amount of aluminum that is allowed in a vaccine is 0.85mg/dose (or 850mcg). However, there’s some important information missing when it comes to understanding why this amount was chosen.
Where does this number come from?
This dose was scientifically determined to be the amount of aluminum which is most effective when used as an adjuvant in vaccines. This is the amount of aluminum which was found to trigger an effective, sustained immune response to the injected antigen. This chosen amount is not based on safety. In fact, in the ATSDR document, there are zero references to any published scientific research on why the 0.85mg limit is considered safe.
Stated a different way, there is no experimental scientific evidence of any kind that proves this amount of aluminum is safe to inject.
Why is that?
HISTORY
In 1975, the US Food and Drug Administration (FDA) granted “GRAS” status to nine different aluminum compounds, including aluminum hydroxide and aluminum phosphate, two aluminum adjuvants used as ingredients in current vaccines.
“GRAS” = “Generally Recognized as Safe”
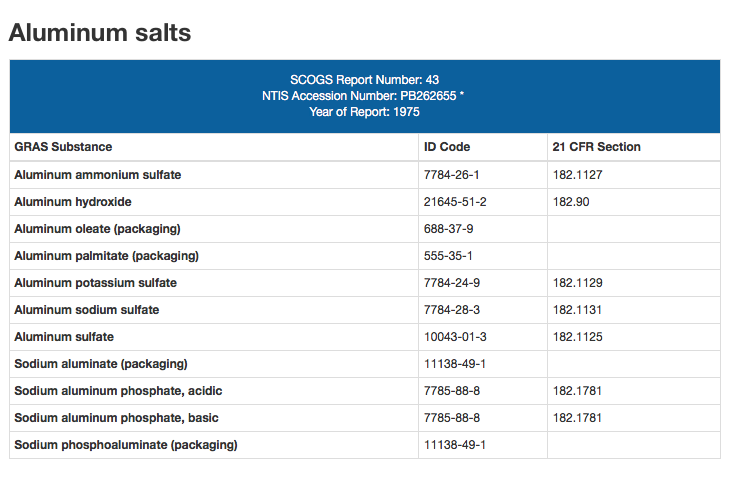
Therefore, since 1975, aluminum has been considered “safe” by the FDA, due to historical use of certain amounts of aluminum in various products. Unfortunately, not only are we relying on pre-1975 science in recognizing the toxicity of aluminum in the body, but we are ignoring the impact of chronic low-dose toxicity, and today we are being bombarded with much more aluminum in our environment (and vaccines) than ever before.
Curiously, if you were to perform a simple search of the scientific literature available on aluminum hydroxide and aluminum phosphate prior to 1970, you can find studies such as this one, where it is being used to produce experimental epilepsy and seizures in monkeys. Or this one, where it was found to cause neurofibrillary degeneration of nerve cells (which is known to lead to the development of Alzheimer’s). Or this one, in which it was described that there were “difficulties” in mass-vaccinating children with aluminum-containing vaccines, due to febrile reactions, aluminum cysts at the site of injection, post-vaccination encephalopathy (brain dysfunction, disease, or disorder), paralytic poliomyelitis of the injected limb, and other unfavorable results…
There’s an endless amount of research on experimentation with aluminum for various purposes, but when it comes to real information on what might be a safe limit of injection, the literature is lacking.
DETERMINING A SAFE LIMIT
In the study of toxicology, the method for finding a safe limit is to first experimentally determine the acute (short term/one-time dose) and chronic (long term low dose) No Observed Adverse Effect Level (NOAEL) or NOEL for a given substance, via a specified route (dermal, inhaled, ingested, or injected – each route will give widely varying results). An aluminum compound would be administered to a test subject (e.g. “lab rats”), and scientists would perform a health risk assessment. Any observed effects compared to the control group would be noted. The dose would be reduced until no adverse effects were observed. Using that exposure level, a margin of safety would be applied by reducing the dose by a factor of 10, 100, or 1000. This would help account for variation in interspecies differences (from rats to humans, for example), and also provide extra protection for our most sensitive population, infants and pregnant women. (This is a simplified explanation of the process.) The resulting exposure limit deemed “safe” would be much smaller than the highest dose at which no adverse effects were observed.
When it comes to injecting aluminum into the body, none of this has ever been done. No experimental studies have been performed to determine a NOAEL, and therefore no safe limit or maximum allowable dose level (MADL) has been elucidated.
[If anyone can find scientific research on this, please alert me to its existence.]
What scientific research or evidence do we have for an applicable “safe” limit?
In the FDA Code of Federal Regulations on TPN therapy (Total Parenteral Nutrition – the feeding of a person intravenously), it states:
“premature [newborns], who receive parenteral levels of aluminum at greater than 4 to 5 [micro]g/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity.”
And that,
“Tissue loading may occur at even lower rates of administration.”
Based on the above, it can be gathered that an exposure level of even 5 micrograms of aluminum (per kilogram body weight, per day) is a dose that would cause observable adverse effects in infants. This dose is much higher than what an experimentally determined safe limit would be. However, this is the closest thing that we have to a potential injectable reference dose for how much is approaching “unquestionably too much”.
Some calculations:
The average newborn weighs approximately 7.5lbs or 3.5kg.
“Safe limit” = 5 micrograms/kg/day
Multiply by weight of newborn: (5mcg/kg/day)(3.5kg) = 17.5 micrograms/day.
Amount of aluminum in the hepatitis B vaccine, given on the first day of life = 250 micrograms.
Demonstrably, the amount of aluminum in just one hepatitis B vaccine is over 14x the “safe limit” for how much a newborn would receive in one day.
More calculations:
The average 2 month old weighs approximately 11lbs or 5kg.
“Safe limit” = (5 micrograms/kg/day) (5kg) = 25 micrograms/day.
There are several vaccines administered at 2 months of age, according to the CDC schedule.
Diptheria, tetanus, pertussis. (DTaP) / Polio (IPV) / Haemophilus b (HIB) / Hepatitis B / Pneumococcal (PCV) / Rotavirus
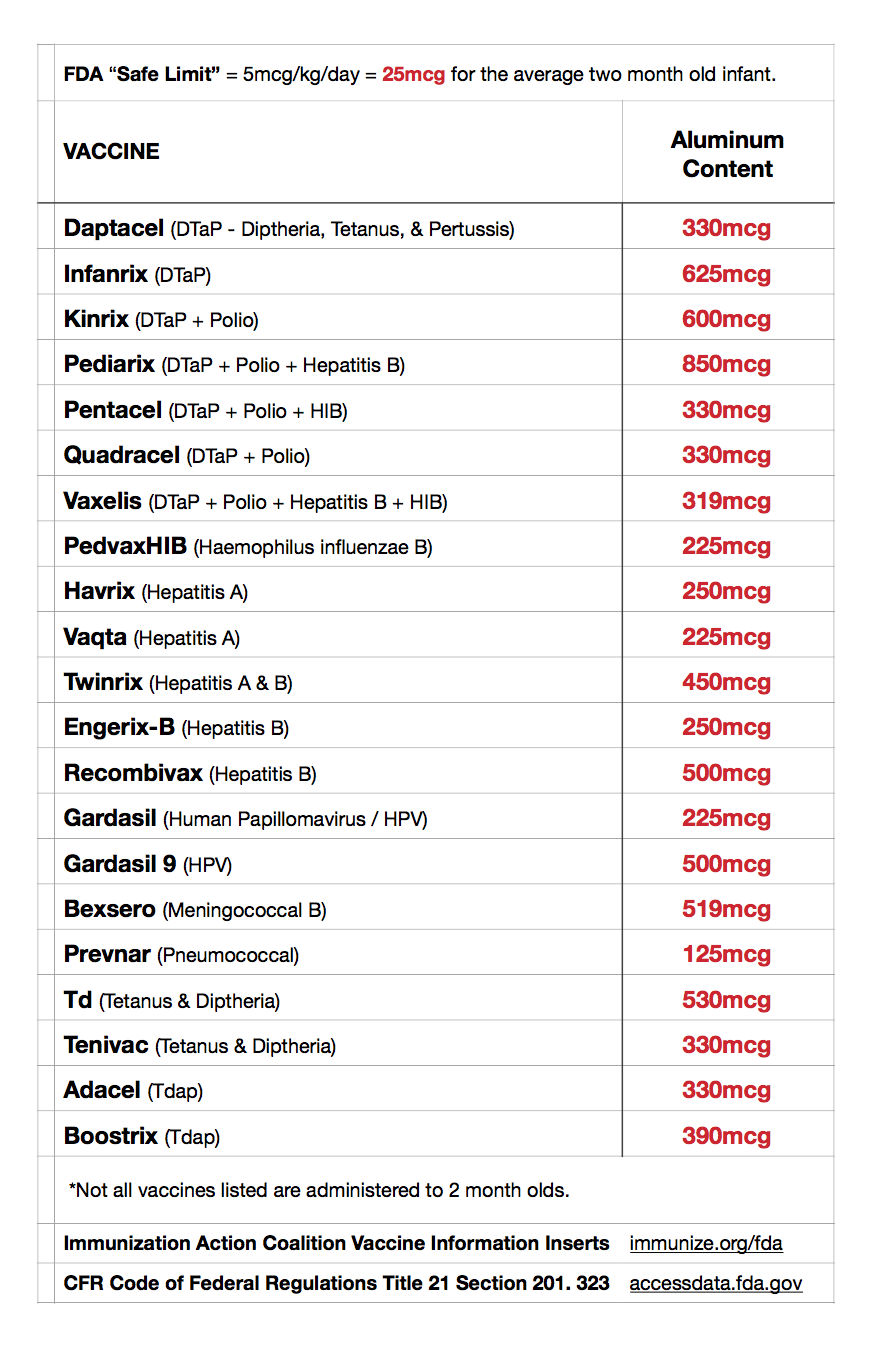
Vaccines containing aluminum that may be administered at a 2 month appointment include:
Pediarix: DTaP, IPV, HepB (850mcg), PCV (125mcg), PedvaxHIB (225mcg)
850mcg + 125mcg + 225mcg = 1200mcg aluminum.
The “safe limit” for a two month old in one day, according to the FDA = 25mcg.
Injected amount via vaccines recommended by the CDC = 1200mcg.
Therefore, the amount of aluminum that may be administered to a 2 month old infant in one day, exceeds the safe limit set by the FDA by 48x.
The CDC schedule recommends this same set of vaccines at 4 months and 6 months, adding another vaccine for influenza 6 months.
Even when adjusting for an exposure level of zero micrograms for the days between a child’s 2, 4, and 6 month visits, the long-term or chronic safe limit is still exceeded. At 25mcg/day from 2 months of age to 6 months of age (120 days), the safe limit of exposure would be 3000mcg. (1200mcg)(3 sets of vaccines, one set for each visit) = 3600mcg. (Technically, this comparison is not scientifically sound due to how dramatically the safe limit is exceeded in one day.)
*A list of vaccines which contain aluminum is at the end of this article.
AUTOIMMUNITY
While there is no research or experimental scientific data on what is truly a safe limit of aluminum to inject via vaccine, there is quite a bit of research on the adverse health effects of injecting aluminum adjuvants.
In the scientific literature, aluminum adjuvants in vaccines have been linked to the development of various autoimmune diseases, such as rheumatoid arthritis, lupus, thyroid disease or autoimmune thyroiditis, fibromyalgia, chronic fatigue, multiple sclerosis, myocarditis, antiphospholipid syndrome / thrombosis (risk of blood clots), Sjögren syndrome (dry eyes, dry mouth), and more.
It’s well-researched and acknowledged that aluminum-containing vaccines triggers conditions such as “autoimmune (auto-inflammatory) syndrome induced by adjuvants” (ASIA), and “macrophagic myofasciitis” (MMF), which may manifest as any one of the above autoimmune conditions in children or adults, or present as cognitive dysfunction in addition to chronic and increasing inflammation and pain located in joints and muscles.
Dr. Romain Gherardi, a specialist of neuromuscular diseases, has been researching macrophagic myofasciitis and treating it in his hospital clinic in France for several years. His findings continue to be supported through new evidence and research. This is an excerpt from one of his studies, published in 2012:
“Aluminium oxyhydroxide (alum)… has been used in vaccines for its immunological adjuvant effect since 1927. Alum is the most commonly used adjuvant in human and veterinary vaccines, but the mechanisms by which it stimulates immune responses remain incompletely understood.
Although generally well tolerated, alum may occasionally cause disabling health problems in presumably susceptible individuals. A small proportion of vaccinated people present with delayed onset of diffuse myalgia, chronic fatigue and cognitive dysfunction, and exhibit very long-term persistence of alum-loaded macrophages at the site of previous intramuscular (i.m.) immunization, forming a granulomatous lesion called macrophagic myofasciitis (MMF). Clinical symptoms associated with MMF are paradigmatic of the recently delineated ‘autoimmune/inflammatory syndrome induced by adjuvants’ (ASIA). The stereotyped cognitive dysfunction is reminiscent of cognitive deficits described in foundry workers exposed to inhaled Al particles.
Alum safety concerns will largely depend on whether the compound remains localized at the site of injection or diffuses and accumulates in distant organs. Animal experiments indicate that biopersistent nanomaterials taken up by monocyte-lineage cells in tissues, such as fluorescent alum surrogates, can first translocate to draining lymph nodes, and thereafter circulate in blood within phagocytes and reach the spleen, and, eventually, slowly accumulate in the brain.”
Macrophagic myofasciitis: characterization and pathophysiology.
Gherardi RK, et al. Lupus. 2012.
NEUROTOXICITY
Another aspect of aluminum adjuvant toxicity is that the aluminum can remain at the injection site for several years. Not only can this cause persistent itching and contact allergy, but the brain gradually accumulates the injected aluminum. As a result of this long-term biopersistence, autoimmune and neurological conditions have been found to manifest as late as ten years post-vaccination.
It is documented that aluminum adjuvants cause neurological damage. The damage is typically delayed and chronic, once again due to the long-term persistence of aluminum at the injection site. Aluminum destroys motor neurons in the brain and slowly causes a greater and greater amount of neuroinflammation. In one study, the damage most closely resembled Amyotrophic Lateral Sclerosis (ALS).
The neurological damage observed also suggests that aluminum adjuvants may play a significant role in the rising rate of autism. Dr. Russel Blaylock, a neuroscientist who has spent years researching the issue, has actually proposed a mechanism for the development of autism due to immunoexcitotoxicity. (More on the vaccine-autism connection here).
IS ALUMINUM IN VACCINES SAFE?
Aluminum in vaccines is not safe. There was never a valid reason to claim safety. Even at the microgram levels injected, these amounts are harmful and there’s no way to know if you or your child will be significantly affected – weeks, months, or even years later. With the increase in the amount of aluminum-containing vaccines that we are expected to receive, the incidence of autoimmune and neurological disease is rising alongside of it. More and more children are developing chronic, debilitating conditions. The independent scientific research on aluminum adjuvants causing autoimmunity and neurotoxicity – is only growing – and there is less and less doubt that aluminum-containing vaccines have caused chronic illness in a significant portion of the population. It’s a tragedy that scientific research continues to validate that aluminum is in fact causing Alzheimer’s disease, and yet doctors and nurses urge the elderly to get their aluminum-adjuvanted pneumonia vaccine every year.
“Everyday there is more evidence that this relationship is more than casual. In humans, adjuvants can induce non-specific constitutional, musculoskeletal or neurological clinical manifestations and in certain cases can lead to the appearance or acceleration of an autoimmune disease in a subject with genetic susceptibility.”
Adjuvants- and vaccines-induced autoimmunity: animal models.
Ruiz JT, et al. Immunol Res. 2017.
There is so much more scientific research on the adverse health effects of aluminum. A small amount of research hilighted here, in relation to vaccine adjuvants. I would encourage you to go to PubMed.gov and search the literature for yourself.
*Note: If you want to argue that the consumption of food and breastmilk contaminated with aluminum is a greater concern than aluminum in vaccines, please read the following article and understand that toxicity depends on route of exposure (ingestion, inhalation, dermal, or injection): Why You Cannot Compare the Amount of Aluminum in Breastmilk to Vaccines.
Also, I highly suggest watching the documentary, Injecting Aluminum.
Aluminum-containing vaccines.
Vaccine package inserts provide the information as listed below:
Additional References:
Overview of basic toxicological principles: http://ec.europa.eu/health/ph_projects/2003/action3/docs/2003_3_09_a21_en.pdf
The spectrum of ASIA: ‘Autoimmune (Auto-inflammatory) Syndrome induced by Adjuvants’: http://journals.sagepub.com/doi/full/10.1177/0961203311429316
Clinical features in patients with long-lasting macrophagic myofasciitis.
Rigolet M, et al. Front Neurol. 2014. https://www.ncbi.nlm.nih.gov/m/pubmed/25506338/
A role for the body burden of aluminium in vaccine-associated macrophagic myofasciitis and chronic fatigue syndrome.
Exley C, et al. Med Hypotheses. 2009. https://www.ncbi.nlm.nih.gov/m/pubmed/19004564/
Atypical presentation of macrophagic myofasciitis 10 years post vaccination.
Ryan AM, et al. Neuromuscul Disord. 2006. https://www.ncbi.nlm.nih.gov/m/pubmed/17005400/
[Lessons from macrophagic myofasciitis: towards definition of a vaccine adjuvant-related syndrome].
Gherardi RK. Rev Neurol (Paris). 2003. https://www.ncbi.nlm.nih.gov/m/pubmed/12660567/
Macrophagic myofasciitis lesions assess long-term persistence of vaccine-derived aluminium hydroxide in muscle.
Gherardi RK, et al. Brain. 2001. https://www.ncbi.nlm.nih.gov/m/pubmed/11522584/
Aluminum adjuvants of vaccines injected into the muscle: Normal fate, pathology and associated disease: http://www.sciencedirect.com/science/article/pii/S1286011516000254
Severe manifestations of autoimmune syndrome induced by adjuvants (Shoenfeld’s syndrome): https://link.springer.com/article/10.1007/s12026-016-8811-0
Strong evidence linking and Alzheimer’s: https://www.hippocraticpost.com/mental-health/strong-evidence-linking-aluminium-alzheimers/