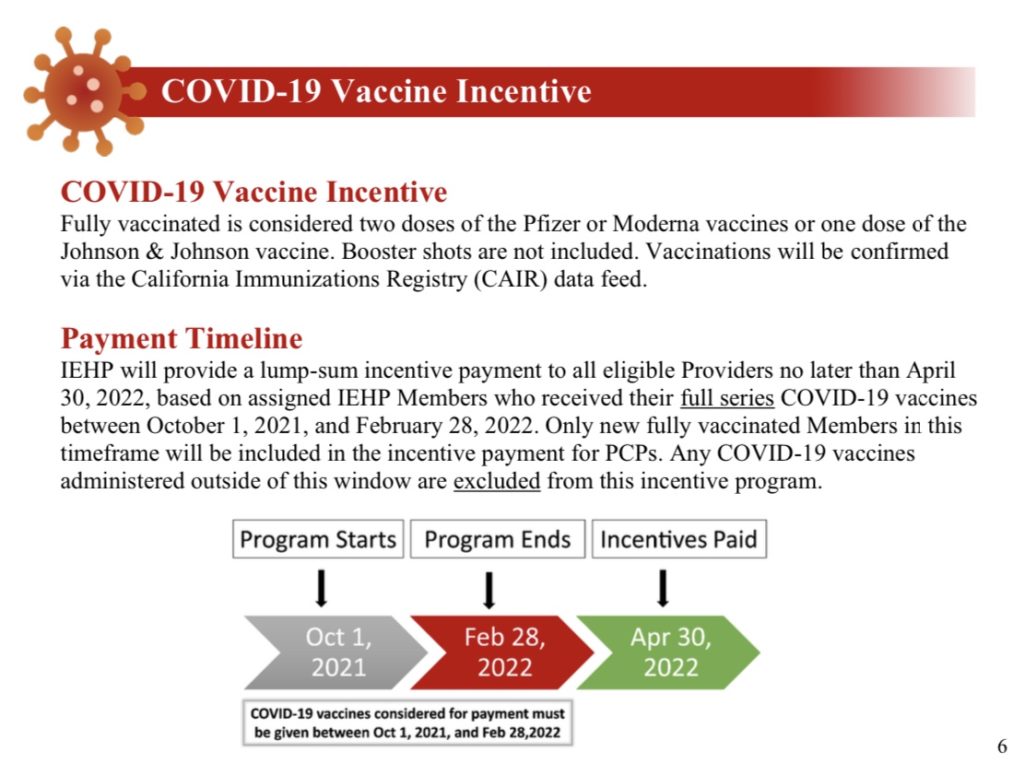
“IEHP will provide a lump-sum incentive payment to all eligible Providers no later than April 30, 2022, based on assigned IEHP Members who received their full series COVID-19 vaccines between October 1, 2021, and February 28, 2022.”
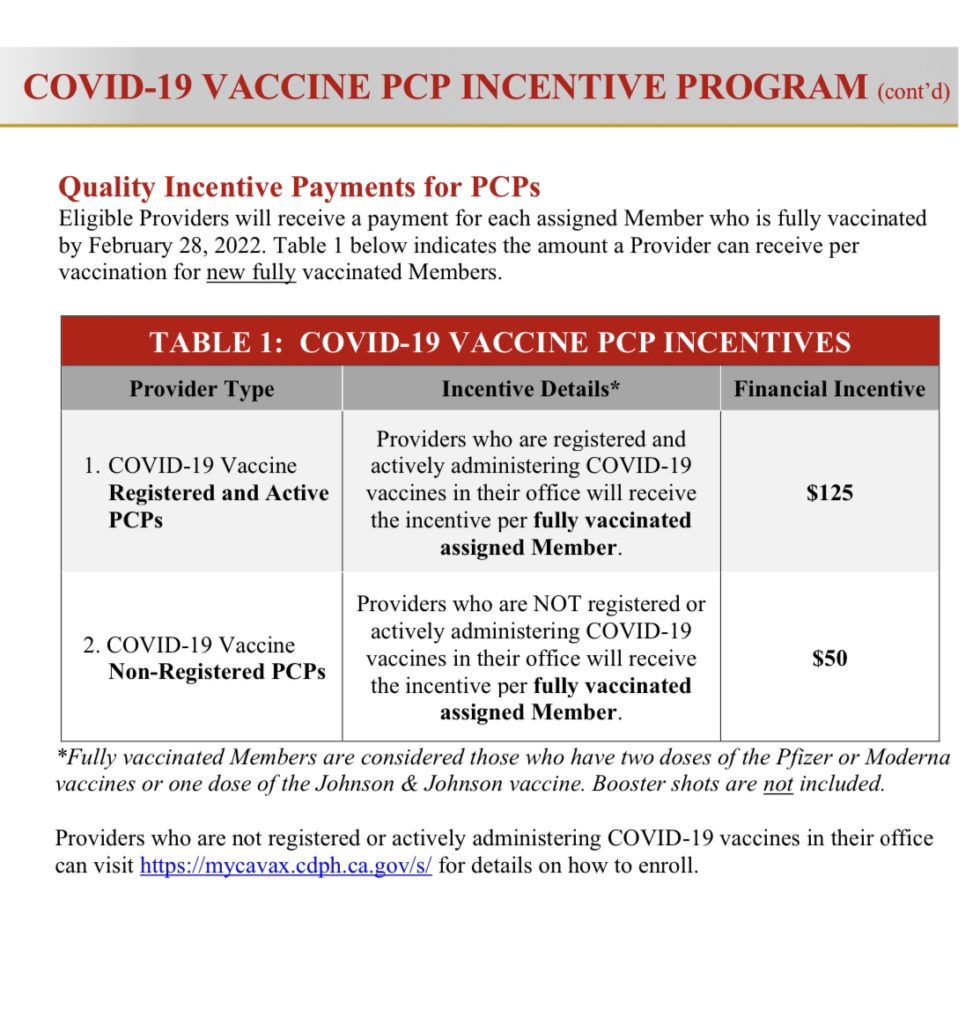
Doctors will receive $125 per fully vaccinated patient who is a Medi-Cal member.
Source: IEHP.org
Physician letter written to CDC and FDA regarding serious adverse events in vaccinated patients, in September, was ignored. Law firm Siri & Glimstad send follow up letter requesting immediate response:




A few of the patients and events described:
“An otherwise healthy patient under age 40 developed low back pain and had an episode of urinary incontinence after receiving a Covid-19 vaccine. The day after the second dose, the patient felt numbness and tingling down one leg. The symptoms rapidly progressed such that a few days later, patient was admitted to the hospital for bilateral leg paralysis. MRI showed transverse myelitis. Weekly follow-up imaging showed that the process continued to worsen and ascend, despite maximal medical therapy. Eventually patient became quadriplegic, blind and had a tracheostomy placed. Patient developed autonomic dysfunction (irregular heart rate and hypotension) and became cognitively impaired.”
“A generally healthy patient in the early seventies, with no smoking history or prior lung disease, received a Covid-19 vaccine and developed generalized malaise with a poor appetite and a new cough. According to the spouse, patient lost >15 lbs during this time period. The cough worsened over the course of the next month and the patient was hospitalized. CT scan of the chest showed bilateral diffuse ground-glass opacities, typical of COVID pneumonia. However, patient was COVID negative on repeated testing. Patient clinically deteriorated and required intubation. Bronchoscopy with aiveolar iavage was positive for Pneumocystis Pneumonia,a rare opportunistic infection which typically only afflicts the severely irnrnunosuppressed such as AIDS ortransplant patients. Patient developed rnuiti organ system faiture.”
“A man in his eariy sixties received the Covid-19 vaccine and developed dizziness which worsened overtime, He had no smoking history and was othewise healthy. On the day of hospital admission, patient experienced sudden neurologic deterioration and required intubation for airway protection. Imaging studies ofthe head showed cerebral venous sinus thrombosis. CVST is a very rare type of stroke,estimated by Johns Hopkins to occur 5 per mililon per year, with a female to male ratio Of 3:1. Over 85% of the patients had at least one identifiable risk factor, such as prothrombotic state, use of oral contraceptives, malignancy Or infectiono My patient had zero risk factors, other than the fact that he had been vaccinated against COVID-19.”
Source: Siri & Glimstad
“Here we show that in convalescent individuals who had experienced mild SARS-CoV-2 infections (n = 77), levels of serum anti-SARS-CoV-2 spike protein (S) antibodies declined rapidly in the first 4 months after infection and then more gradually over the following 7 months, remaining detectable at least 11 months after infection.”
“Overall, our results indicate that mild infection with SARS-CoV-2 induces robust antigen-specific, long-lived humoral immune memory in humans.”
Source: Nature

“…one large, well-designed study found remdesivir modestly reduced the time to recover from COVID-19 in hospitalized patients with severe illness. A few smaller studies found no impact of treatment on the disease whatsoever. Then, on 15 October… the fourth and largest controlled study delivered what some believed was a coup de grâce: The World Health Organization’s (WHO’s) Solidarity trial showed that remdesivir does not reduce mortality or the time COVID-19 patients take to recover.”
“…on 22 October, the U.S. Food and Drug Administration (FDA) approved remdesivir for use against the pandemic coronavirus SARS-CoV-2 in the United States—the first drug to receive that status.”
“In late August it noted a disproportionately high number of reports of liver and kidney problems in patients receiving remdesivir compared with patients receiving other drugs for COVID-19.”
“FDA never consulted a group of outside experts that it has at the ready to weigh in on complicated antiviral drug issues.”
Source: Science
CDC’s definition of “unvaccinated” includes individuals which have received a vaccine:

“Persons were considered fully vaccinated ≥14 days after receipt of the second dose in a 2-dose series (Pfizer-BioNTech or Moderna COVID-19 vaccines) or after 1 dose of the single-dose Janssen (Johnson & Johnson) COVID-19 vaccine; partially vaccinated ≥14 days after receipt of the first dose and <14 days after the second dose in a 2-dose series; and unvaccinated <14 days after receipt of the first dose of a 2-dose series or 1 dose of the single-dose vaccine or if no CAIR2 vaccination data were available.” [Emphasis mine.]
“COVID-19–associated hospitalizations were defined as hospital admissions occurring ≤14 days after a first SARS-CoV-2 infection. COVID-19–associated deaths were defined as deaths occurring ≤60 days after the date of a first laboratory-confirmed SARS-CoV-2 infection or deaths with COVID-19 listed as a cause of or contributing condition to death.”
Source: CDC